.webp)
© History Oasis
In 1968, two Procter & Gamble chemists tried to create an easily digestible fat for premature babies.
Fred Mattson and Robert Volpenhein mixed sucrose with fatty acids and created something unexpected: a molecule so large it passed straight through the human body undigested. They were trying to help sick infants and instead created something the body couldn't absorb at all.
They called it Olestra.

In 1998, Frito-Lay launched WOW! chips nationwide. They positioned the product as real potato chips with zero fat. You could eat an entire bag and consume no fat calories. The secret was olestra, a synthetic molecule that tasted like fat but passed through the body undigested.
Frito-Lay put olestra in their most popular flavors: Lay's, Ruffles, Doritos, Tostitos.
First-year sales hit $400 million.
Americans bought the dream of guilt-free snacking.
But guilt-free came with a messy cost.

Every bag carried an FDA-mandated label: "This Product Contains Olestra. Olestra may cause abdominal cramping and loose stools."
The molecule was large enough to escape digestion entirely. When people ate significant amounts, the undigested fat passed through quickly. The technical term is steatorrhea. The common term is urgent diarrhea.
The label also noted added vitamins A, D, E, and K. Olestra stripped these fat-soluble vitamins from the body as it exited. Frito-Lay had to fortify chips to prevent nutritional deficiencies from snacking.

Online forums exploded with stories. People described eating a bag during road trips and needing emergency bathroom stops. Others reported accidents. The phrase "anal leakage" became permanently associated with the brand.
Frito-Lay insisted the stories were exaggerated. They funded studies showing most people experienced no symptoms. The FDA eventually agreed and removed the warning requirement in 2003. But the damage was done.
Sales dropped by half between 1998 and 2000. By 2004, Frito-Lay quietly rebranded WOW! chips as "Lay's Light" and removed olestra from most products.
By 2016, they discontinued the chips line entirely.

Frito-Lay misjudged human psychology. People wanted permission to eat chips without guilt, but not if it meant digestive consequences. The warning label created a mental association: these chips cause problems. Even if most people never experienced symptoms, the possibility was enough.
The company also underestimated how much people valued the ritual of chip eating. A bag of WOW! chips tasted nearly identical to regular chips but felt different psychologically. If the fat was fake, was the pleasure fake too?

Procter & Gamble, which created olestra and licensed it to Frito-Lay, had spent $500 million developing the product over 28 years. They built a dedicated factory in Cincinnati. When sales collapsed, they sold the factory to Twin Rivers Technologies in 2002 and stopped pursuing new food applications for olestra.
Frito-Lay never disclosed their total investment in WOW! chips, but industry estimates put marketing and production costs in the hundreds of millions. They reformulated their entire snack line, built new production systems, and launched a national advertising campaign. All for a product that lasted less than two decades.
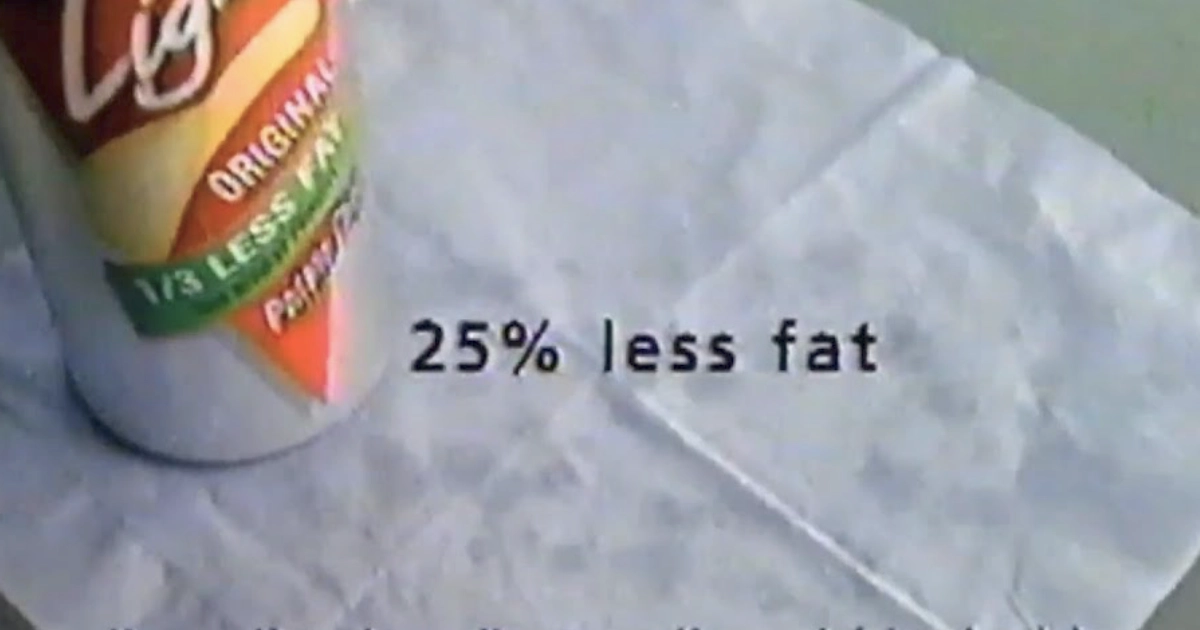
Kellogg's Pringles Light used olestra until 2015. They positioned it differently: a reduced-fat option, not a zero-fat miracle. The product survived longer than WOW! chips but eventually disappeared for the same reason. No company wanted to defend chips associated with bathroom emergencies.

As of 2025, no olestra products are sold in the United States. The European Union and Canada banned it. The molecule exists now only in industrial applications—deck stains and lubricants, sold under the brand name Sefose.
WOW! chips promised consequence-free indulgence. The warning label told a different story.